Choose the best clinical strategy with the help of our seasoned clinical drug development experts.…

Medical device
Experienced in the regulatory requirements for clinical investigations of medical and in-vitro diagnostic devices, we assist you in designing, implementing and managing a clinical development program for your medical device (pre- and post-marketing).
THE PATH FROM DESIGN TO MARKETING OF
A NEW MEDICAL DEVICE (MD OR IVDMD)
CAN BE BLURRED AND COMPLEX
Regulations and requirements vary by region, by country, level of risk, product-specific claims and intended use.


With a comprehensive experience in all types of medical devices, our MD team includes:
- Project managers
- Clinical monitors
- Data managers
- Biostatisticians
- Regulatory and QA experts
- Medical writers
They can assist you:
- in designing, implementing, and managing a clinical development program (pilot/proof of concept, confirmatory, Post-Marketing Clinical Follow-up)
- regardless of the type of medical device (MD, IVDMD, “combined” device (MD + drug), connected MD, software as a medical device)
- whether you aim at supporting a pre-marketing regulatory submission (i.e. CE mark package for a Notified Body), supporting product reimbursement, or monitoring post-market product use (Post-Marketing Clinical Follow-up / PMCF).
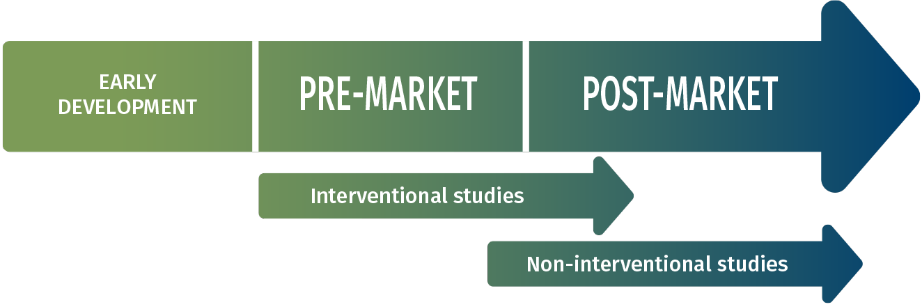
INTERVENTIONAL STUDIES
- Pilot studies
- Confirmatory studies (Pre and Post Market)
- Innovation Post-Authorisation Safety Studies (PASS)
NON INTERVENTIONAL STUDIES
- PMCF studies
- Post-marketing registries
- Health economic assessments
- Materiovigilance reporting
- Patient centered studies
- Health Systems database
- Analyses (SNDS…)
OUR SERVICES
CONSULTING IN MEDICAL DEVICE DEVELOPMENT
- Literature reviews
- Clinical and regulatory strategy
- Trial design and validation
- Product / Risk classification
- Early feasibility studies
- Risk management
PRE- / POST-MARKETING CLINICAL INVESTIGATIONS
- Design and medical writing
- Regulatory submissions
- Clinical investigation management and follow-up
- Biometry and data sciences
- Safety and PSUR
- Clinical study reports
ICTA’s experience encompasses Medical Devices, IVDMDs, “combined” MDs, and software as medical device, used in many indications across a large range of therapeutic areas
THE ICTA RWE TEAM CAN ALSO ASSIST YOU IN DESIGNING
PRE-MARKETING STUDIES
to better understand the use of the medical device in the larger clinical setting and for regulatory decision making
POST-MARKETING STUDIES
for labelling-CE-mark expansion/variation and post-market surveillance (real world studies)
Regardless of the stage of development of your medical device, the ICTA team offers insight, expertise and know-how to help you:
Understand the market and identify the right MD strategy
Set up the overall MD clinical development plan
Assess the safety and performance, and gather data for CE mark/registration
Mitigate risk and keep your MD compliant with regulations and ISO requirements
OTHER AREAS OF EXPERTISE