Secure collaborative platform
I-SIT®, the scalable, web-based integrated IT platform to connect the dots for a secure e-management
I-SIT®, WEB-BASED SECURE IT COLLABORATIVE PLATFORM
ICTA manages clinical information with a built for purpose system, i-SIT® (ICTA Secure Information Technology), a web-based secure IT platform which offers a streamlined approach to optimise the management of your projects.
i-SIT is a common, centralised and unified platform that is robust enough to support the various information management workflows to help harmonise and improve collaboration, ensure compliance and data consistency.
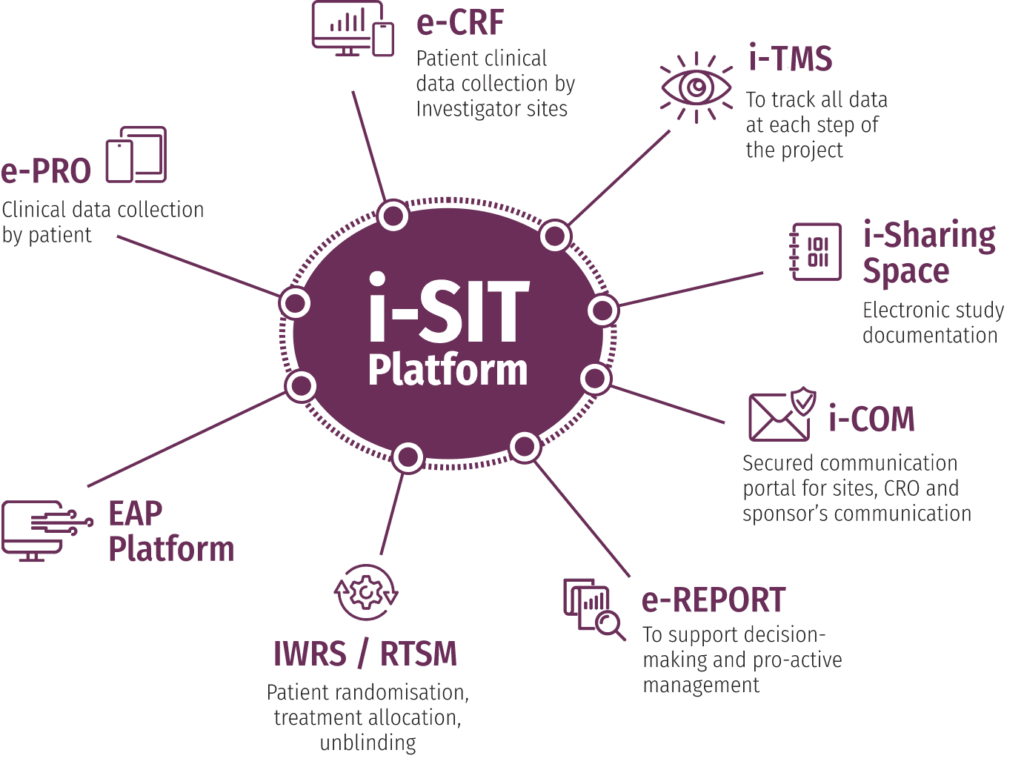
It is made up of a range of integrated IT tools which provide an end-to-end support to optimize project management, decision making, data collection, processing and analysis. It is deployed according to the requirements of the project and accessible to all stakeholders with specific accesses.
It is designed to address the challenges inherent in modern clinical trials such as access, interoperability between systems, and frustrating technology experiences.
DESIGNED
to optimize the management of local / international projects (GCP and ISO standards), regardless of the type of project
DEVELOPED
in close collaboration with the operational teams using the Agile methodology to meet clients’ various requirements, and progressive releases
VALIDATED
in compliance with ICH, 21 CFR part 11, ISO 27001 and 9001, FDA, RGPD using ICTA documentation issued from GAMP5 and built up with an expert in validation of computerized systems
HOSTED
on ICTA servers or HDS servers when required
I-SIT, COLLABORATIVE PLATFORM TO EFFICIENTLY MANAGE CLINICAL PROJECTS AND GENERATE MEANINGFUL DATA
No software
installation
100%
web-based
21 CRF part 11 and
GAMP5 compliance
Data
Privacy
I-SIT IS PRAISED BY OUR CLIENTS FOR:
its customised design which allows a quick study setup and a short lead time to start collecting data
Thanks to the standard kernel of our systems and after analysis of current practices
its modular architecture which enables a flexible, scalable and customisable configurability
to handle all types of international and local clinical trials, clinical investigations, Early Access/Compassionate Programs, Unlike rigid off the shelf Clinical Trial Management Systems on the market
its user friendliness and consistency for all stakeholders
Our system of workflows and automatic alerts provide an intuitive and efficient experience for all trial stakeholders
IT support in house
to address clients’ requests in collaboration with the clinical operations and biometrics departments
IT expertise
Always using innovative and state-of-the-art technologies
Better analytics
We house all data in one central location, offering ease of use in data analysis
